|
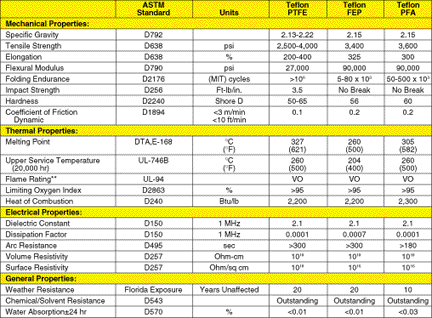
PFA Fluorocarbon Information PFA Fluorocarbon Resin Table 1
*Typical values are unsuitable for specifications. Properties were measured at 23 degreesC (73 degreesF), unless otherwise noted. **Statements regarding behavior in a flame situation are not intended to reflect hazards presented by this or any other material when under actual fire conditions. The following characteristics contribute to the unique properties of PFA fluorocarbon resins: • Nonpolarity:The carbon backbone of the linear polymer is completely sheathed by the electron cloud of fluorine atoms, much like a wire core is protected by insulation coating. This ensheathment, and the angles at which the carbon-fluorine bonds are disposed, causes the centers of electronegativity and electropositivity to be perfectly balanced across the polymer chain cross section. As a result, no net charge difference prevails. This nonpolarity of the polymer is partly responsible for its lack of chemical reactivity. • Low interchain forces: The bond forces between two adjacent polymer chains are significantly lower than the forces within one chain. PTFE linear polymer chains are otherwise restrained. However, in FEP and PFA, interpolymer chain entanglement of the pendant structure precludes the shifting of polymer chains to relieve the implied load. The "creep" normally associated with PTFE is mostly avoided with FEP and even more so with PFA. • High C-F and C-C bond strengths are among the strongest in single bond organic chemistry. The polymer must absorb considerable energy to disrupt these bonds. Chemical reactions represent a kinetic and thermodynamic resolution of bond-making and bond-breaking in favor of the most stable system. These bond strengths are hard to overcome. • Crystallinity:The high degree of crystallinity in these semicrystalline polymers results in high melting points, mechanical properties, and an integral barrier to migrating, small, nonpolar molecules. Under certain conditions, these molecules penetrate the plastics. • High degree of polymerization: The unbranched nature of the polymers and their low interpolymer chain attraction requires very long chain lengths in PTFE and entanglement in FEP and PFA to provide load-bearing mechanical properties. The chain length also has an impact on flow and crystallinity of the polymers. These unique properties lead to the following benefits: • High melting points (327°C [621°F] for PTFE; 260°C [500°F] for FEP, and 305°C [582°F] for PFA). The melting point of PTFE is one of the highest in organic polymer chemistry. Other materials can attain higher temperatures, but they degrade rather than melt. Compared to PTFE, the lower melting temperature of FEP results from lower°of polymerization and crystallinity. In PFA, a higher degree of polymerization, enhanced entanglement of the pendant structure, and lower comonomer content combine to provide a melting point closer to that of PTFE. • High thermal stability: Due to the strength of the carbon-fluorine and carbon-carbon single bonds, appreciable thermal energy must be absorbed by the polymers before thermal degradation. The rate of decomposition of a part of PFA depends on the particular resin, temperature, and heat exposure time; and to a lesser extent, pressure and nature of the environment. At maximum continuous service temperatures, thermal degradation of the resins is minimal. For example, at 400°C, FEP is measured at 4/100,000 of 1 percent, and PTFE at 1/100,000 of 1 percent. At high processing temperatures, adequate ventilation is recommended. • High upper service temperature (260°C [500°F] for PTFE, 204°C [400°F] for FEP and 260°C [500°F] for PFA). The polymers' high melting points and morphological features allow components made from the resin to be used continuously at the stated temperatures. Above this temperature, the component's physical properties may begin to decrease. The polymer itself, however, will be unaffected if the temperature is insufficient for thermal degradation. • Insolubility: There is no known solvent for PFA fluorocarbon resins under ordinary conditions. •
Inertness to chemical attack:The intrapolymer-chain bond
strengths preclude reaction with most chemicals. Under relatively
unusual circumstances the polymer can be made to react. Examples
of unusual reagents include: • Low coefficient of friction: The low coefficient of friction of PFA results from low interfacial forces between its surface and another material and the comparatively low force to deform. • Low dielectric constant and dissipation factor: PFA provides low, if not the lowest, values for these parameters. These low values arise from the polymer's nonpolarity as well as the tight electron hold in the ultrapolymer bonds. • Low water absorptivity: For PFA to absorb water, the surface must remain wet for a long enough time for water to become physico-chemically associated with the polymer chains, and then it must become included in the polymer bulk structure. Water is a very high energy material and PFA has a very low surface energy. Therefore, these events are energetically incompatible and only occur under special circumstances and to a small extent. • Excellent weatherability: Weather includes light of various wavelengths (IR, visible, UV), water (liquid or gas), other gases, and normal temperatures and pressure. The physical and chemical makeup of PFA makes it inert to these influences. • Flame resistant:PFA will burn when exposed to flame, but will not continue to burn when the flame is removed. • Excellent toughness: Some mechanical properties of PFA resins are shown in Table 1. Toughness characteristics are high and differ somewhat between resin types. Tefzel
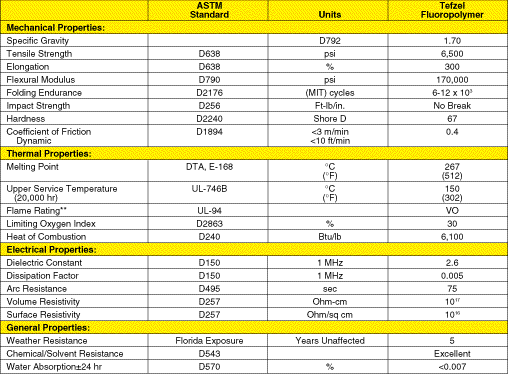
Fluoropolymer Resin However, the presence of hydrogen or of hydrogen and chlorine sacrifices chemical and thermal stability. For example, in simple molecules, the C-H bond is ~5 percent weaker than the C-F bond, and the C-C1 bond is 25 percent weaker. In addition to weak chemical bonds, the arrangement of the substituting elements along the polymer chain has a marked effect on the resin's chemical stability. In this regard, solubility can be a leading indicator. Tefzel, with a regularly alternating structure of the monomers tetrafluoroethylene and ethylene, has no known solvent in ordinary conditions. In contrast, polyvinylidene fluoride, the chemical isomer of Tefzel, is soluble in common industrial ketones (e.g., methyl ethyl ketone). Ethylene/trifluoroethylene is soluble in some fluorinated solvents. The substituted polymers are also adversely affected by strong acids and alkalies. Of the three mentioned, Tefzel is compatible with the broadest range of chemicals under a wide range of conditions. Equal proportions of the comonomers react to produce a polymer where individual monomers alternate regularly along its chain. Typical properties of Tefzel appear in Table 2. Data in Table 2 and Table 1 show that the polarity and accompanying interpolymer chain attraction enhance the physical properties of the substituted polymer over those of the unsubstituted, fully fluorinated polymer. Note, for example, that Tefzel has about 1.5 times greater strength than PFA and 2 times greater stiffness. Tefzel
Fluoropolymer
Summary
and Conclusion |